Submissions
Submission Preparation Checklist
As part of the submission process, authors are required to check off their submission's compliance with all of the following items, and submissions may be returned to authors that do not adhere to these guidelines.- The contribution is original and unpublished, and it is not under review or submitted for publication in another journal. Otherwise, it must be justified in "Comments to the Editor."
- The files for submission are in Microsoft Word, OpenOffice, or RTF format (provided they do not exceed 6 MB).
- URLs for the references have been provided when available.
- The text is double-spaced; uses a 12-point font; employs italics instead of underlining (except for URLs); and figures and tables are embedded in the text, not at the end of the document as appendices.
- The text adheres to the style standards and bibliographic requirements outlined in the Author Guidelines section under About the Journal.
- In the case of submission to a section with peer review (e.g., articles), the instructions available in Ensuring a Blind Peer Review have been followed.
Author Guidelines
Quick Tips for Authors
Instructions for Authors and Journal Policies
- 1. Aims & Scope
- 2. Editorial Freedom
- 3. Platinum Open Access
- 4. Submission Policies
- 5. Article Types and Requirements
- 6. Editorial Policies and Ethical Standards
- 6.1 Peer Review
- 6.2 PJS Express (Fast Track)
- 6.3 Principles for Publication of Research Involving Humans
- 6.4 Use of Animal in Research
- 6.5 Clinical Trial Registration
- 6.6 Standards for Reporting
- 6.7 Research and Publication Integrity
- 6.8 Data Sharing and Accessibility
- 6.9 Copyright
- 6.10 Authorship and Responsibilities
- 6.10.1 Ethical Responsibilities of Authors
- 6.10.2 Authorship
- 6.10.3 Acknowledgments
- 6.10.4 Group Authorship
- 6.10.5 Role of Medical Writer
- 6.10.6 Corresponding Author
- 6.10.7 Author Contributions
- 6.10.8 Author Identifiers (ORCID)
- 6.10.9 Deceased or Incapacitated Authors
- 6.10.10 Correction to Authorship
- 6.10.11 Potential Conflicts of Interest for Authors
- 6.10.12 Confidentiality
- 6.11 Role of the Funding Source
- 6.12 Cover Letter
- 6.13 Mandatory Forms and Signatures
- 6.14 Advertising Policy
- 7. Manuscript Preparation Guidance
- 8. After Acceptance
- 9. Publication Process After Acceptance
- 10. Post Publication
- 11. Supplement Publication Policy
The Portuguese Journal of Surgery (PJS)
1. Aims & Scope
The Portuguese Journal of Surgery (PJS) is the official organ of the Portuguese Society of Surgery, which has been published continuously since 2007 and is published quarterly. Supplementary issues on selected themes may also be published at the discretion of the Editor-in-Chief.
The Portuguese Journal of Surgery (PJS) is a peer-reviewed, open-access journal dedicated to advancing the field of surgery through the dissemination of high-quality, evidence-based research. The journal aims to serve as a global platform for exchanging cutting-edge knowledge, innovative techniques, and multidisciplinary approaches that enhance patient care and surgical practice.
Key Objectives:
- Promote Scientific Research: To publish original research articles that address fundamental and clinical aspects of surgery, ranging from basic science to translational research.
- Foster Innovation: To highlight advancements in surgical techniques, minimally invasive procedures, robotic surgery, and emerging technologies that contribute to better outcomes.
- Encourage Best Practices: To disseminate clinical guidelines, case reports, and systematic reviews that inform and refine surgical practices across a wide range of specialties.
- Facilitate Global Collaboration: To serve as a forum for knowledge exchange among surgeons, researchers, and healthcare professionals worldwide, encouraging a diversity of perspectives and collaborative research efforts.
- Focus on Patient-Centered Care: To emphasize research that prioritizes patient safety, outcomes, and quality of life through innovative approaches to surgical care and postoperative management.
Scope:
The Portuguese Journal of Surgery (PJS) welcomes submissions covering, but not limited to, the following areas:
- Colorectal
- Endocrine
- Gastrointestinal
- Hepatopancreatobiliary
- Oncology
- Transplant surgery
- Traumatology
- Vascular
- Bariatric and Metabolic Surgery
The PJS journal also accepts submissions on topics such as:
- Perioperative Care of the Surgical Patient
- Minimally Invasive and Robotic Surgery: Advancements in laparoscopic, endoscopic, and robotic techniques.
- Surgical Education and Training: Articles that focus on the improvement of surgical training, professional development, and mentoring.
- Ethics and Outcomes Research: Studies addressing the ethical implications of surgical care, as well as patient outcomes and healthcare policy.
PJS encourages submissions of various formats, including original research articles, review articles, systematic reviews, case reports, guidelines, study protocols, letters, and expert opinions. The journal also supports the publication of video demonstrations of new techniques to enhance learning and understanding.
Abstracting and Indexing Information
All articles published in PJS are included in:
- RCAAP - Repositório Científico de Acesso Aberto de Portugal
PJS complies with the Uniform Requirements for Manuscripts Submitted to Biomedical Journals produced by the ICMJE (International Committee of Medical Journal Editors). Please see http://www.icmje.org.
2. Editorial Freedom
The PJS adopts the definition of editorial freedom of the International Committee of Medical Journals Editors (ICMJE) described by the World Association of Medical Editors, establishing that the Editor-in-Chief assumes full authority over the journal's editorial content. The Portuguese Society of Surgery, as the owner of the PJS, does not interfere in the process of evaluation, selection, programming, or editing of any manuscript, and the Editor-in-Chief has full editorial independence.
3. Platinum Open Access
PJS is a platinum open-access journal without author fees (APC - article processing charges). All articles published by PJS are made freely and permanently accessible online immediately upon publication, without charges.
4. Submission Policies
Studies must be scientifically valid, following standards, relevant to the field, and written in good English. Submission of a manuscript requires that the authors have complied with all policies in this document.
Submission to PJS implies that the content has not been published before, in any language, in whole or in part, except as a brief abstract in the proceeding of a scientific meeting; and the manuscript is not under consideration for publication elsewhere; that its publication has been approved by all co-authors, as well as by the responsible authorities. The PJS will not be held legally responsible should there be any compensation claims.
4.1. Preprint Policy
PJS accepts articles previously published on preprint servers. PJS will consider for peer review, articles previously available as preprints. If the article is accepted for publication, authors are requested to update any pre-publication version with a link to the final published article.
4.2. Permissions
Authors wishing to include figures, tables, or text passages that have already been published elsewhere are required to obtain permission from the copyright owner(s) and to include evidence that such permission has been granted when submitting their manuscript. Any material received without such evidence will be assumed to originate from the authors.
4.3. Duplicate Submission or Prior Publication
During submission, authors must state that neither the manuscript nor any significant part of it is under consideration for publication elsewhere or has appeared elsewhere in a manner that could be construed as a duplicate or prior publication of the same, or similar, work. Abstracts for scientific meetings are not considered previous publications but should be cited in the Acknowledgments section of the manuscript. Posting of un-refereed manuscripts to a community preprint server by the author will not be considered before publication; see the Preprints policy section above for additional information.
All articles must be submitted through our submission system. Submit manuscript here.
Please read the instructions and make sure to download the required files that must be signed and sent along with your article: here.
4.4. Declarations
- Cover Letter
- Ethics Approval
- Consent for Publication
- Conflict of Interests statement
- Authors' contribution statement
- Acknowledgements
- PJC License Agreement
- Personal Communications
- Use of Copyright-Protected Material
Before submitting a manuscript, authors must prepare the following documents:
- The Cover Letter (template available here) should be written and signed by the corresponding author and include the relevant data to justify the article’s publication and its originality. Moreover, the cover letter must state that the manuscript has not been submitted to any other journal than the PJS, has not been previously published, adheres to the structure and style of the PJS, complies with ethical and legal guidelines, and indicates sources of funding (if applicable).
- The Authors' Contributorship Statement (template available here) should be filled out by the corresponding author and signed by all authors, specifying the contribution of each author and his/her responsibility for the data validity of the article’s content. Finally, each author should confirm the copyright policy.
- Conflicts of Interest Disclosure (ICMJE template, available here). Authors must declare any potential conflicts of interest that could cause a bias (or be seen as a bias) in the conduct of their work in the individual ICMJE template declaration. Consequently, authors must disclose all financial and personal relationships related to the submitted work. They should also identify any benefits associated with the article’s publication, including stock or economic interests in companies or other institutions, wages or awards, grants or other forms of funding, consulting, patent rights, or other financial relations. The existence of conflicts of interest in publishing an article does not constitute a reason for rejection, as long as the said conflicts are appropriately declared. For any queries on what constitutes a relevant financial or personal interest, the authors should contact the Editor-in-Chief.
When applicable, authors should also submit:
- Consent for Publication
- Informed Consent of each participant
- Authorization to Reproduce/use (copyright-protected material) previously published material (for example Figures)
- Declaration of Approval by the Ethics Committees of the institutions involved
- Acknowledgments
- Personal Communications
All documents must be provided during the submission process, preferably through the online platform here.
5. Article Types
Manuscripts are submitted to blind peer review by at least two anonymous reviewers, except where otherwise stated. Final acceptance or rejection rests with the Editor-in-Chief and/or the Deputy Editors, who reserve the right to reject any material for publication.
Manuscripts should be written in a clear, concise, direct style so that they are intelligible to the reader, including physicians from other specialties and the general public. When contributions are considered suitable for publication based on scientific content, the Editor-in-Chief reserves the right to modify the texts to remove ambiguity and repetition and to improve communication between authors and the readers. In the case that significant changes are required, the manuscript will be returned to the author for revision.
Manuscripts that do not comply with the instructions for authors may be returned for modification before being reviewed.
The PJS publishes the following types of articles:
- Editorials
- Review Articles
- Systematic Reviews and Meta-analyses
- Original Articles
- Case Reports
- Images in Surgery
- Letters to the Editor
- Study Protocols
- Current Perspectives
- Methods papers
Authors should indicate in the cover letter what type of manuscript is being submitted for publication.
6. Editorial Policies and Ethical Standards
6.1. Peer Review
Peer review is the system used to assess the quality of a manuscript before it is published. Independent researchers in the relevant research area assess submitted manuscripts for originality, validity, and significance to help the Editor-in-Chief determine whether the manuscript should be published in the journal. Manuscripts should be written in a clear, concise, and direct style. In addition, they must not have been published or submitted for publication elsewhere.
Reviewers must respect confidentiality and are not allowed to reveal details of any manuscript in the peer-review process. If reviewers wish to involve a colleague in the review process, they should first obtain permission from the editor. Reviewers who choose to use AI-assisted technologies to support the review process must declare their use to the editorial team and are responsible for ensuring that any AI-generated content incorporated into the review is accurate and unbiased.
All research articles, and most other article types, published in the PJS go through a peer-review process. Letters to the Editor or Editorials are evaluated by the Editorial Board but may require an external review.
After evaluating the manuscript, it can be:
- Accepted without changes
- Accepted but contingent on minor modifications
- Revaluated after major modifications
- Rejected
The manuscript is initially reviewed by the Editor-in-Chief and can be rejected at this stage without being sent to the reviewers. The primary acceptance criteria are quality, clarity, and originality. If a manuscript does not comply with the instructions for authors, it can be rejected before being reviewed. Final acceptance or rejection rests with the Editor-in-Chief or, in the event of a conflict of interest on his part, with one of the Deputy Editors, who reserves the right to refuse any material for publication.
The timeline for this process has been defined as follows:
- Upon receipt of the article, the Editor-in-Chief or one of the Deputy Editors, will send the manuscript to at least two reviewers provided that it complies with the publication standards and fits the editorial policy. It may be rejected at this stage, without being sent to reviewers.
- The reviewers should respond to the Editor within no more than two weeks with their comments on the manuscript, in addition to any suggestions regarding the acceptance or rejection of the work.
- Upon receiving the reviewer’s comments, a first decision will be taken by the Editorial Board within no more than 10 days, either (i) accepting the article for publication with no modifications, (ii) sending the reviewers’ and editor’s comments to the Authors to proceed as indicated, or (iii) rejecting the article.
- The Authors have 10 days to submit the revised version of the manuscript, complying with the modifications recommended by the experts and the Editorial Board. This version should be uploaded to the PJS website, under the same editorial process (i.e., keeping the same ID# as used in the submission), with the changes highlighted in a different color, in addition to a new Supplementary Document answering all the questions raised.
- The Editor-in-Chief has 15 days to decide on the new version, either (i) rejecting, (ii) accepting the new version of the article, or (iii) submitting it to one or more external reviewers whose opinions may or may not coincide with those resulting from the first revision.
- In the case that the manuscript is sent back for external review, the experts have two weeks to send their comments and their suggestions regarding the acceptance or rejection for publication.
- After analysis of the reviewers’ suggestions, the Editor-in-Chief (i) may accept the article with this new version, (ii) reject it, or (iii) request further revisions. In this event, the Authors have 20 days to submit a revised version, which may undergo a further review process by external experts, if the Editor-in-Chief so determines.
The editor’s final decision for acceptance-rejection of a submitted paper is based on the following criteria:
- Originality: original subject and/or method, with valuable information and presentation of new results or confirmation of previously verified results.
- Timeliness and/or novelty: a topic that is on the agenda of scientific meetings or communications or is a new subject.
- Relevance: applicability of the results to solve real issues in clinical practice.
- Innovation and relevance: advances in scientific and technical knowledge and/or in clinical practice.
- Reliability and scientific validity: high methodological quality.
- Presentation: good writing and text organization (good logical coherence and presentation of the material).
Even though the Editors and Reviewers make every effort to ensure the technical and scientific quality of the manuscripts, the final responsibility for the content (namely the accuracy of the observations as well as the opinions expressed) is the sole responsibility of the Authors.
6.2. PJS Express (Fast-Track)
PJS Express is a fast-track publication system available for urgent and important manuscripts that meet PJS requirements for expedited review and publication. Fast-track publication can be requested through the manuscript submission process, clearly indicating the reason why the manuscript should be considered for expedited review and publication. The Editorial Board will decide whether the manuscript is suitable for expedited publication and will communicate its decision within 48 hours. If the Editor-in-Chief finds the manuscript unsuitable for expedited publication, the manuscript may be proposed for the normal review process or the authors may withdraw their submission. The editorial decision on manuscripts accepted for expedited review will be made within five working days. If the manuscript is accepted for publication, the PJS will aim to publish it ahead of print within 15 days.
6.3. Principles for Publication of Research Involving Human Subjects
6.3.1. Ethics Approval
When reporting a study that involved human participants, their data, or biological material, authors should include a statement that confirms that the study was approved (or granted exemption) by the appropriate institutional and/or national research ethics committee (including the name of the ethics committee and reference number) and certify that the study was performed in accordance with the ethical standards as laid down in the Helsinki Declaration of the World Medical Association updated in 2013.
If a study was granted an exemption from requiring ethics approval, this should also be detailed in the manuscript (including the reasons for the exemption). Manuscripts must:
- Include a statement on ethics approval and consent (even where the need for approval was waived)
- Include the name of the ethics committee that approved the study and the committee's reference number
Declarations of research ethics compliance must appear in the Methods section of Original Investigations.
Examples of statements to be used when ethics approval has been obtained:
- All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration updated 2013. The study was approved by the Ethics Committee of the …(No. ...).
- This study was performed in line with the principles of the Declaration of Helsinki 2013. Approval was granted by the Ethics Committee of ..(Date.../No. ...).
6.3.2. Ethics Approval for Case Studies
Authors of Case Reports should follow their institution's policies about whether ethics approval is required. If such approval is obligatory, the manuscript must include a statement about ethics review/approval. Most institutions will have specific policies on this subject. Since they typically include detailed case descriptions, Case Reports are generally considered to contain identifying information and therefore publication consent must be obtained. Authors should be aware to secure informed consent from the individual (or parent or guardian if the participant is a minor or incapable). See also section on Patient Protections / Informed Consent.
6.3.3. Consent for Publication
Whenever possible, and regardless of the article type, authors should avoid including any information identifying individual patients or participants. If identifying information is necessary (including any individual details, images or videos), the patient (or legal representative) must be shown the manuscript and sign a written publication consent.
All presentations of case reports must have consent for publication.
Authors can use the PJS form (Patient Consent for Publication in PJS), or may use another form that contains equivalent elements. In submission, authors will be required to attest that a signed form has been obtained and to provide a copy of the form.
In cases where images are entirely unidentifiable and there are no details on individuals reported within the manuscript, consent for publication of images may not be required. The final decision on whether consent to publish is required lies with the Editor-in-Chief.
Exceptions where it is not necessary to obtain consent:
- Images such as x-rays, laparoscopic images, ultrasound images, pathology slides unless there is a concern about identifying information in which case, authors should ensure that consent is obtained.
6.4. Use of Animals in Research
Any experiments involving animals must be demonstrated to be ethically acceptable and where relevant conform to national guidelines for animal usage in research.
6.5. Clinical Trial Registration
The World Health Organization (WHO) definition of a clinical trial is "any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes". The WHO defines health interventions as "A health intervention is an act performed for, with or on behalf of a person or population whose purpose is to assess, improve, maintain, promote or modify health, functioning or health conditions" and a health-related outcome is generally defined as a change in the health of a person or population as a result of an intervention.
To ensure the integrity of the reporting of patient-centered trials, authors must register prospective clinical trials (phase II to IV trials) in suitable publicly available repositories. For example www.clinicaltrials.gov or any of the primary registries that participate in the WHO International Clinical Trials Registry Platform.
The trial registration number (TRN) and date of registration should be included as the last line of the manuscript abstract.
For clinical trials that have not been registered prospectively, authors are encouraged to register retrospectively to ensure the complete publication of all results. The trial registration number (TRN), date of registration, and the words "retrospectively registered" should be included as the last line of the manuscript abstract.
6.6. Standards for Reporting
PJS advocates complete and transparent reporting of research. Authors are recommended to adhere to the reporting guidelines hosted by the EQUATOR Network when preparing their manuscript.
Checklists are available for several study designs, including:
- CONSORT checklist for Randomized Clinical Trials
- STROBE checklist for Observational Studies
- PRISMA or MOOSE checklist for Systematic Reviews and Meta-Analyses – interventional and observational studies
- SPIRIT or PRISMA-P checklist for Study Protocols
- STARD checklist for Diagnostic Accuracy Studies
- COREQ checklist for Qualitative Studies
- CHEERS checklist for Economic Evaluation of Health Interventions
- STARI checklist for Implementation Studies
- SQUIRE checklist for Standards for Quality Improvement Reporting Excellence
- STREGA checklist for Associations between Genetic Factors and Clinical Outcomes
- CARE checklist for Case Reports
6.7. Research and Publication Integrity
PJS considers irresponsible and unethical research practices to include fabrication (invention of data), falsification (tampering with data, including images), misrepresentation (plagiarism, duplicate publication, misattribution), intentional failure to disclose conflict of interests (COIs), or any other behavior that lessens the reliability or integrity of the research record.
PJS takes seriously its responsibility to respond to suspicions or allegations of misconduct according to its misconduct handling policy.
For all Original Investigations, authors have a responsibility to report methodology accurately, clearly, and with sufficient detail such that the findings can be independently confirmed, and to retain the underlying data for at least 5 years after study completion unless questions have been raised regarding the conduct of the research, in which case all relevant data must be retained until all such matters are resolved. Collectively, the authors are responsible for ensuring that the article is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
6.8. Data Sharing and Accessibility
In recognition of the increased attention given to the reproducibility of research findings, and to enhance opportunities for research collaboration, each manuscript reporting a clinical trial must include a data-sharing statement.
PJS does not currently have a particular data-sharing expectation (beyond the stipulation that data be available for editors’ inspection, as detailed in the Research and Publication Integrity section); the requirement is simply that authors be transparent about their data-sharing intentions. Data sharing statements should specify the type of data that will be shared (e.g., deidentified, individual participant data underlying the results presented in the manuscript); whether other documents will be available (e.g., study protocol, statistical analysis plan, analytic code); if data will be available, the start and end dates of this availability; with whom data will be shared (e.g., anyone, researchers with a methodologically sound proposal); the types of analyses to be allowed (e.g., any, meta-analysis); and the procedure for requesting access.
Authors are encouraged to review the table in the ICMJE's publication regarding data sharing for further detail on the type of information to be included in data-sharing statements and the possible wording of such statements. Clinical trials that began enrolling participants on or after January 1, 2019, should include a data-sharing plan when registering the trial and should update the registry record if the plan is subsequently modified.
6.9. Copyright
Articles in PJS are licensed under a Creative Commons Attribution Non-Commercial License (CC BY-NC 4.0).
- Authors who publish with PJS agree to the following terms: Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-NC 4.0) that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors may share and distribute their article on non-commercial websites and repositories immediately upon publication, with an acknowledgment of its initial publication in this journal.
6.10. Authorship and Responsibilities
6.10.1. Ethical Responsibilities of Authors
PJS policies generally follow those provided in ICMJE Recommendations https://www.icmje.org/recommendations and the Core Practices of Committee on Publications Ethics (COPE) https://publicationethics.org.
The recommendations stipulated by the Committee on Publication Ethics regarding the use of artificial intelligence in scientific research writing, as well as on the attribution of authorship of the manuscripts as established, will be strictly followed.
The use of AI and AI-assisted technologies in scientific writing
Where authors use AI and AI-assisted technologies in the writing process, these technologies should only be used to improve the readability and language of the work and not to replace researcher tasks such as producing scientific insights, analyzing and interpreting data, or drawing scientific conclusions. Such writing assistance should be disclosed in a statement at the end of the article in the acknowledgment section. Applying these technologies should only be done with human oversight and control, and authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased.
Authors who have used AI technology in any part of their study, enhancing search strategies, or in the development of review articles must describe its use in the Methods section in sufficient detail to enable replication of the approach including the tool used, version, and prompts where applicable. Authors should not list AI and AI-assisted technologies as authors or co-authors, nor cite AI as an author. Authors are ultimately responsible and accountable for the originality, accuracy, and integrity of the work.
Important note: The PJS may use software to screen for plagiarism.
Authors must follow the rules of good scientific practice, which include:
- The manuscript should not be submitted to more than one journal for simultaneous consideration.
- The submitted work should be original and should not have been published elsewhere in any form or language (partially or in full) unless the new work concerns an expansion of previous work.
- A single study should not be split up into several parts to increase the number of submissions and submitted to various journals or to one journal over time (salami-slicing).
- Results should be presented clearly, honestly, and without fabrication, falsification or inappropriate data manipulation (including image manipulation).
- No data, text, or theories by others are presented as if they were the author’s own (‘plagiarism’). Proper acknowledgments to other works must be given (this includes material that is closely copied (near verbatim), summarized and/or paraphrased), quotation marks (to indicate words taken from another source) are used for verbatim copying of material, and permissions secured for material that is copyrighted.
- Authors should make sure they have permission for the use of software, questionnaires, surveys, and scales in their studies (if appropriate).
- Reuse of Copyrighted Material: Any text, figure, table, or data from other sources must be clearly attributed. For all borrowed material, authors are responsible for applying for permission from the relevant publisher(s) for rights and are responsible for paying any permissions fees. In addition to providing proof of permission to the editorial office, authors must include appropriate wording in the figure legend or a table note to indicate the source of the material.
- Research articles and non-research articles (e.g., Perspective, Review) must cite appropriate and relevant literature in support of the claims made.
- Excessive and inappropriate self-citation or coordinated efforts among several authors to collectively self-cite is strongly discouraged.
- Authors are strongly advised to ensure the author group, the Corresponding Author, and the order of authors are all correct at submission.
- Upon request, authors should be prepared to send relevant documentation or data to verify the validity of the results presented.
- Authors must correct mistakes once they discover a significant error or inaccuracy in their published article. The author(s) is/are requested to contact the journal and explain in what sense the error is impacting the article. A decision on how to correct the literature will depend on the nature of the error. This may be a correction or retraction. The retraction note should provide transparency on which parts of the article are impacted by the error.
- At the time of submission, authors must declare whether artificial intelligence (AI) – assisted technologies (such as Large Language Models [LLMs], chatbots, or image creators) in the production of submitted work. Authors who use such technology should describe, in both the cover letter and the submitted work, how they used it. Chatbots (such as ChatGPT) should not be listed as authors because they cannot be responsible for the accuracy, integrity, and originality of the work, and these responsibilities are required for authorship. Therefore, humans are responsible for any submitted material that includes the use of AI-assisted technologies. Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased. Authors should not list AI and AI-assisted technologies as an author or co-author, nor cite AI as an author. Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI. Humans must ensure there is appropriate attribution of all quoted material, including full citations.
If there is suspicion of misbehavior or alleged fraud, the PJS will carry out an investigation following COPE guidelines. If, after investigation, there are valid concerns, the author(s) concerned will be contacted under their given e-mail address and allowed to address the issue. Depending on the situation, this may result in the Journal’s implementation of the following measures, including, but not limited to:
- If the manuscript is still under consideration, it may be rejected and returned to the author.
- If the article has already been published online, depending on the nature and severity of the infraction:
- An erratum/correction may be placed with the article
- An expression of concern may be placed with the article
- Or in severe cases, retraction of the article may occur.
The reason will be given in the published erratum/correction, expression of concern or retraction note. Please note that retraction means that the article is maintained on the PJS platform, watermarked “retracted” and the explanation for the retraction is provided in a note linked to the article.
6.10.2. Authorship
The PJS follows the criteria on authorship recommended by the ICMJE Defining the Role of Authors and Contributors (http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html).
Designated authors should meet all four criteria for authorship in the ICMJE Recommendations:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
- Drafting the work or revising it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All those designated as authors should meet all four criteria for authorship, and all who meet the four criteria should be identified as authors. Those who do not meet all four criteria should be acknowledged.
Any potential authorship disputes brought to the editors’ attention will be handled in line with COPE guidelines.
6.10.3. Acknowledgments
Any individual who does not qualify as an author but who contributed to the work described in the manuscript must be named in the Acknowledgment. In particular, if medical writer(s)/editor(s) have been involved, their role must be explicitly acknowledged, and their affiliation/source of funding must be listed. Authors may also express thanks or note assistance in the Acknowledgements.
Authors should obtain permission to acknowledge from all mentioned in the Acknowledgments.
6.10.4. Group Authorship
If authorship is attributed to a group (either solely or in addition to 1 or more individual authors), all members of the group must meet the full criteria and requirements for authorship as described above, and all group member authors must complete Authorship Forms. If all members of a group do not meet all authorship criteria, a group must designate 1 or more individuals as authors or members of a writing group who meet full authorship criteria and requirements and who will take responsibility for the group. Group names should appear at the end of the byline and should not be interspersed within the list of individually named authors. Group authors may not be included for article types with limited numbers of authors (e.g., opinion articles).
6.10.5. Role of Medical Writer
If a medical writer was involved in the creation of the manuscript, PJS needs a signed statement from the corresponding author to include their name and information about the funding of this person:
- This information should be added to the Acknowledgments and/or Contributors section.
- We require signed statements from any medical writers declaring that they have permitted to be named as an author, as a contributor, or in the Acknowledgments section.
6.10.6. Corresponding Author
One author is assigned as the Corresponding Author and acts on behalf of all co-authors as the preferred correspondent with the editorial team during the submission and review process. Any author can be a corresponding author, but only one author can perform this role.
The corresponding author is responsible for the following requirements:
- Ensuring that submission requirements are met and submitting the manuscript to the journal
- Ensuring that all listed authors have approved the manuscript before submission, including the names and order of authors
- Managing all communication between the Journal and all co-authors, before and after publication
- Providing transparency on the re-use of material; making sure disclosures, declarations, and transparency on data statements from all authors are included in the manuscript as appropriate
- Sending corrections and ensuring that the final version of the paper to be published is approved by all the authors
Third-party submissions
All manuscripts must be submitted by an author and may not be submitted by a third party.
6.10.7. Author Contributions
PJS asks all authors to specify their individual contributions. A brief description of the contribution of each individual listed as an author must be provided. Link Authors Contributorship statement
Examples of such statement(s) are:
- All authors contributed to the study conception and design. All authors read and approved the final manuscript.
- AA, BB and CC: Material preparation, data collection and analysis.
- DD: Writing the original draft.
- EE: Statistical analysis.
- FF: Conceptualization and supervision.
For more information consult CRediT (Contributor Roles Taxonomy).
6.10.8. Author Identifiers (ORCID)
PJS strongly encourages every author to register for and use an ORCID iD (a persistent digital identifier) that distinguishes each researcher from others with similar names when submitting an article for consideration or acquiring an ORCID ID via the submission process. An ORCID iD connects an author’s affiliations, grants, publications, peer review, and more to ensure recognition for all contributions.
Additional information is available at https://orcid.org/.
6.10.9. Deceased or Incapacitated Authors
For cases in which a co-author dies or is incapacitated during the writing, submission, or peer-review process, and the co-authors feel it is appropriate to include the author, co-authors should obtain approval from a (legal) representative who could be a direct relative.
6.10.10. Correction to Authorship
It is the corresponding author’s responsibility to ensure that the list of authors is correct as far as the online submission form and the submitted text are concerned. Any changes to the list of authors, including the removal or addition of authors that occur between the initial submission and the acceptance for publication will require a written agreement of all the authors, and reasons for these changes in authorship should be explained. New authors are required to confirm their full compliance with PJS authorship criteria. Approval of the change during revision is at the discretion of the Editor-in-Chief.
Changes of authorship (addition or removal) are not accepted after acceptance of a manuscript.
Please note that author names will be published exactly as they appear on the accepted submission! Please make sure that the names of all authors are present and correctly spelled, and that addresses and affiliations are current.
In the case of an authorship dispute during peer review or after acceptance and publication, the PJS will not be in a position to investigate or adjudicate. Authors will be asked to resolve the dispute themselves. If they are unable, the PJS reserves the right to withdraw a manuscript from the editorial process or, in case of a published paper, raise the issue with the authors’ institution(s) and abide by its guidelines.
6.10.11. Potential Conflicts of Interest for Authors
PJS conflict of interest (COI) policies generally follow those of the ICMJE Recommendations. Authors must disclose all relationships that could be viewed as potential COI. A COI may exist when financial or personal relationships with other persons or organizations may inappropriately influence or bias actions. There is a potential for a COI whether or not an individual believes that a relationship affects his or her scientific judgment. COIs can occur as the result of financial relationships, personal and family relationships, or academic competitive pressures.
Editors may use information in COI disclosures as the basis for editorial decisions.
6.10.12. Confidentiality
Authors should treat all communication with the PJS as confidential, which includes correspondence with direct representatives from the Journal such as Editors-in-Chief and/or Handling Editors and reviewers’ reports unless explicit consent has been received to share information.
6.11. Role of the Funding Source
- All sources of funding should be declared as an acknowledgment at the end of the text.
- At the end of the Methods section, under the subheading “Role of the funding source,” authors must describe the role of the study sponsor(s), if any, in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
- If there is no Methods section, the role of the funding source should be stated as an acknowledgment. If the funding source had no such involvement, the authors should so state.
6.12. Cover Letter
Cover letter (template available here), written and signed by the corresponding author, who must justify the publication of the article on the PJS; point out that the article is original, was only submitted for publication on the PJS and not previously published; mention that the manuscript adheres to the structure and style standards adopted by the PJS; point out that the work complies with ethical and legal guidelines (recommendations of the Helsinki Declaration of the World Medical Association and has been evaluated and approved by the Ethics Committee, in the case of an original study); and indicate sources of funding.
6.13. Mandatory Forms and Signatures
PJS requests these forms in the manuscript submission:
- Authors’ Contribution statement
- Conflicts of Interest statements (ICMJE forms)
- Statements of role, if any, of medical writer or editor
- Acknowledgments—written consent of cited individual
- Consent for Publication
- Cover Letter
- PJS License Agreement
- Personal communications—written consent of cited individual
- Use of Copyright-Protected Material—signed permission statements from author and publisher
These statements can be scanned and submitted electronically with your submission. Please note that PJS will accept hand-signed and electronic signatures.
6.14. Advertising Policy
The journal sponsors are pharmaceutical industry companies or other companies that generate revenue through advertising. Other expenses are supported by the Portuguese Society of Surgery.
Advertising cannot influence the scientific independence of the journal or editorial decisions and must conform to the general and specific health care and medicines legislation.
Editorial independence is crucial to scholarly publishing, and the editorial team has full authority to decide on the content of the journal. The criteria for editorial decision-making regarding journal content do not include any perceived effect on advertising revenue. The editors have the right to review all new advertising proposed for the PJS and may reject any advertising deemed not in keeping with the journal’s mission.
7. Manuscript Preparation Guidance
7.1. Language
The title, abstract and keywords must be presented in English and Portuguese. Manuscripts submitted to PJS must be written in Portuguese (from Portugal) and/or English at a reasonable level.
7.2. General Requirements
Manuscripts that do not follow the instructions for authors can be returned for modification before being revised. The file must be saved in the native format of the word processor. The text should be in a single-column format. To avoid unnecessary errors, you are strongly advised to use the grammar and spelling-checking functions of your word processor:
- Manuscripts should be sent in DOC, DOCX format, and should not be blocked or protected.
- The text of the manuscript should be typed double-spaced. Do not format the text in multiple columns.
- The texts must be formatted in the letter "Arial", size 11. Titles and sub-titles must be marked in bold and size 12.
- All margins should be at least 30 mm.
- All pages should be numbered consecutively in the top right-hand corner, beginning in the title page.
- Specify any special characters used to represent characters that are not on the keyboard.
7.3. Manuscript Structure
Structure of the manuscript:
Front page (separate page):
- Title: Title (concise and objective, preferably with less than 12 words).
- Authors, affiliations and ORCID: Name of all authors (clinical or professional name) and respective affiliation (department, institution, city) and ORCID iD.
- Corresponding author: Address and e-mail of the corresponding author.
- Ethical Considerations: Indicate whether or not there are conflicts of interest and include information about patient protection.
- Prizes and previous presentations: Prizes and presentations of the study, prior to submission of the manuscript, must be mentioned.
Second Page
- Abstract: Abstract (maximum word count according to the article type). Please minimize the use of abbreviations and do not cite references in the abstract.
- Keywords: Keywords (according to the article type) representing the main content of the article. Keywords should be easily searchable in indexing databases using Medical Subject Headings (MeSH) terminology found at http://www.nlm.nih.gov/mesh/. In the manuscripts that do not require an abstract, the keywords should be presented at the end.
Following pages – Manuscript body
The following pages should include the main text of the article according to the specific sections of each type. After the references, the illustrations should be presented individually on a new page, in the following order: Tables and Figures.
Original Articles
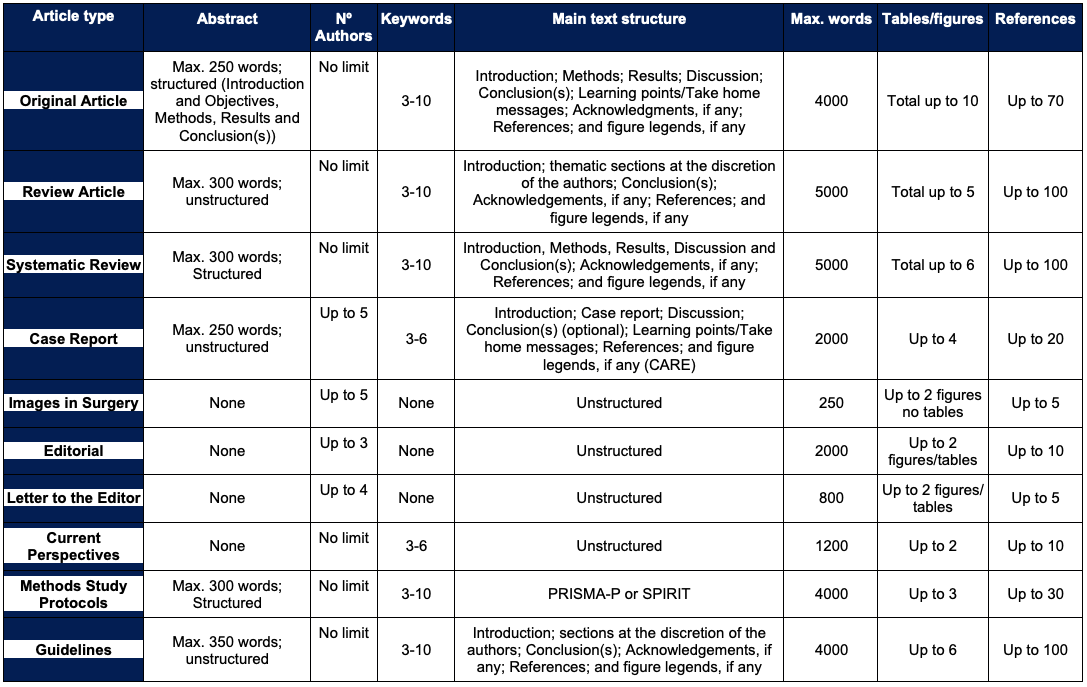
Original Investigation articles (“original articles”) cover areas of clinical or basic research: Clinical trial, Intervention study, Cohort study, Case-control study, Epidemiologic assessment, Survey with high response rate, Cost-effectiveness analysis, Decision analysis, Study of screening and diagnostic tests, Other observational studies – Follow appropriate EQUATOR Reporting Guidelines. They should have a maximum of 4000 words, with a total of up to 10 tables and/or figures, and should be structured as follows:
- Abstract: (maximum 250 words; divided into Introduction, Methods, Results and Conclusion);
- Keywords: 3-10 keywords;
- Main Text: (Introduction; Methods; Results; Discussion; Conclusion);
- Learning points/Take home messages: with bullet points (maximum 100 words);
- Acknowledgements: if any;
- References: (up to 70);
- Figure legends: if any.
Original Article may include Supplemental material with additional relevant supporting figures.
Word limit: Up to 4000
Abstract: Up to 250; Structured
Keywords: 3-10
References: Up to 70
Figures/Tables: Maximum 10 tables/figures
Authors: No limit
Review Articles
Review articles summarize and interpret existing literature on topics of interest to PJS readership. Review Articles should be structured as follows:
- Abstract: (maximum 300 words; unstructured);
- Keywords: 3-10 keywords;
- Introduction;
- Thematic sections: at the discretion of the authors;
- Conclusion(s);
- Acknowledgements: if any;
- References: (up to 70);
- Figure legends: if any.
Word limit: Up to 5000
Abstract: Up to 300; Unstructured
Keywords: 3-10
References: Up to 100
Figures/Tables: Maximum 5 tables/figures
Authors: No limit
Systematic Reviews with or without Meta-Analysis
Systematic Reviews with or without Meta-Analysis must be structured as Introduction, Methods, Results, Discussion and Conclusion(s). The objective of a systematic review should be to produce an evidence-based conclusion. The Methods should give a clear indication of the literature search strategy, data extraction, grading of evidence and analysis. Systematic Reviews should not normally exceed 4000 words, with a total of up to 6 tables and/or figures and up to 100 references. Authors are strongly recommended to consult the PRISMA statement (http://www.prisma-statement.org/), which is intended to help improve the reporting of systematic reviews and meta-analyses. We encourage authors to develop a systematic review protocol (e.g. following PRISMA-P) and register with PROSPERO.
Word limit: Up to 5000
Abstract: Up to 300; Structured
Keywords: 3-10
References: Up to 100
Figures/Tables: Maximum 6 tables/figures
Authors: No limit
Case Reports
Authors should use the CARE guidelines as a guiding framework. Case reports should not exceed 2000 words of body text, with up to 20 references and four tables or figures. They must include abstract (unstructured) and bulleted statements to answer the following questions: What’s already known about this topic? and What does this study add? Learning points/Take-home messages with bullet points are required (maximum 500 words). Please see the Ethics section of the Instructions regarding the preservation of patients’ privacy. Case Reports must have no more than 5 authors.
Word limit: Up to 2000
Abstract: Up to 250; Unstructured
Keywords: 3-6
References: Up to 20
Figures/Tables: Maximum 4 tables/figures
Authors: Up to 5
Learning points/Take-home messages: Up to 500
Images in Surgery
Images in Surgery should have a maximum of 250 words, without Abstract, keywords, tables, or division into sections and up to 5 references may be included.
Word limit: Up to 250
Abstract: None
Keywords: None
References: Up to 5
Figures/Tables: Maximum 2 figures, no tables
Authors: Up to 5
Editorials
Editorials are usually invited opinion pieces, but authors may propose a paper for the Editor-in-Chief consideration.
Word limit: Up to 2000
Abstract: None
Keywords: None
References: Up to 10
Figures/Tables: Maximum 2 tables/figures
Authors: Up to 3
Letters to the Editor
A Letter to the Editor generally takes one of the following forms:
- A substantial re-analysis of a previously published article in the Journal will be considered up to 8 weeks after the publication of the article in question.
- A brief report of cases adequate for the journal's scope and of particular interest to the community.
Word limit: Up to 800
Abstract: None
Keywords: None
References: Up to 5
Figures/Tables: Maximum 2 tables/figures
Authors: Up to 4
Current Perspectives
Perspectives articles address topical issues, controversies, recent scientific developments, or novel observations relevant to any aspect within the broad compass of surgery, from basic science to public policy. They may challenge dogma or present a distinctive point of view. Articles should be timely and engaging. For particularly controversial topics, the Editors may solicit a counterpoint Perspective. Perspectives may be submitted for consideration for publication without an invitation or may be solicited by the Editor-in-chief. Perspectives must not exceed 1250 words and may have no more than ten references and one figure or table. Perspectives should not include an abstract. Manuscripts submitted as Perspectives will be reviewed by the editor-in-chief and may be subjected to additional peer review at the discretion of the editor-in-chief.
Word limit: Up to 1200
Abstract: None
Keywords: 3-6
References: Up to 10
Figures/Tables: Maximum 2 tables/figures
Authors: No limit
Methods Study/Protocol
A study protocol (“methodology manuscript”) describes in detail the plan for conducting a specific clinical study and explains the purpose and function of the study as well as how to carry it out. PJS believes that publishing study protocols will help to improve the standard of surgery research. Study protocols will be published without peer review if the study receives ethics approval and a grant from a major funding body. Any protocols that do not meet both these criteria will be sent for external peer review.
Protocol manuscripts should report planned or ongoing research studies. If data collection is complete, we will not consider the manuscript. We encourage the submission of protocol manuscripts at an early stage of the study.
Study Protocols must abide by the following criteria to be considered for publication:
- Papers must be for proposed or ongoing research and dates must be included in the manuscript. Articles that report work previously completed will not be considered.
- Study protocols must have ethics approval (if applicable).
- All considerations must adhere to the following EQUATOR guidelines: PRISMA-P (Preferred Reporting Items for Systematic review and Meta-Analysis Protocols); SPIRIT (Standard Protocol Items for Randomized Trials).
- Registration is mandatory for any clinical trial as well as for any systematic review and meta-analysis protocols. Approved registries for clinical trials need to meet all of the ICMJE Clinical Trial Registration guidelines. Trial Registration numbers will need to be included in the abstract.
Word limit: Up to 4000
Abstract: Up to 300; Structured
Keywords: 3-10
References: Up to 30
Figures/Tables: Maximum 3 tables/figures
Authors: No limit
Guidelines
Recommendations for clinical practice. This type of article can be submitted by working groups organized within the scope of scientific meetings or associations, or groups of authors with specialized work carried out on the topic in question.
Words: maximum 4000 words (figures and tables)
Abstract: maximum 350 words
Figures / Tables: maximum 6
References: maximum 100
7.4. Style Guide
7.4.1. References
I. Citations in the text
Superscript Arabic numerals are used in the text. Authors may be identified, but the reference number must always be given. References to unpublished data and personal communications should be made directly in the text and should not be numbered. The citation of a reference as “in press” implies that it has been accepted for publication.
Format of the reference list
Make sure that all the references mentioned in the Reference List are cited in the text, and vice-versa. References should be listed using Arabic numerals in the order in which they are cited in the text. The reference list should be added as part of the text, not as a footnote. Reference software-specific reference codes are not allowed.
Make sure that the data provided in the references are correct. When copying references, be careful as they may contain errors. References to published articles should include the name of the first author followed by the names of the other authors, the article title, the journal name and the year of publication, volume and pages. Journal names should be abbreviated according to the Medline style.
A detailed description of the formats of different reference types can be found in “Uniform Requirements for Manuscripts Submitted to Biomedical Journals” (http://www.nlm.nih.gov/bsd/uniform_requirements.html). List all the authors if there are six or fewer. Et al. should be added if there are more than six authors.
It is mandatory to indicate the DOI (Digital Object Identifier) in all references that have it.
Articles Examples
Published article:
- Less or up to 6 authors:
Pietersen PI, Hertz P, Olsen RG, Møller LB, Konge L, Bjerrum F. Transfer of skills between laparoscopic and robot-assisted surgery: a systematic review. Surg Endosc. 2023;37:9030-42. doi: 10.1007/s00464-023-10472-5.
- More than 6 authors:
Gangwani MK, Haghbin H, Priyanka F, Hadi Y, Dahiya DS, Kamal F, et al. Efficacy and safety of EUS-directed transgastric ERCP (EDGE) versus laparoscopic-assisted ERCP: A systematic review and meta-analysis. Endosc Ultrasound. 2024;13:16-21. doi: 10.1097/eus.0000000000000032.
- Article in press:
Maspero M, Sposito C, Mazzaferro V, Ercolani G, Cucchetti A. Cure after surgery for hepato-pancreato-biliary cancers: A systematic review. Dig Liver Dis. 2024 (in press). doi: 10.1016/j.dld.2024.06.021.
Book:
Morrison PJ, Spence RA. Genetics for Surgeons. London: Remedica; 2005.
Book chapter:
Neugut AI, Marvin MR, Chabot JA. Adenocarcinoma of the small bowel. In: Holzheimer RG, Mannick JA, editors. Surgical Treatment: Evidence-Based and Problem-Oriented. Munich: Zuckschwerdt; 2001. p. 82-108.
Web page:
No mínimo, o URL completo deve ser dado e a data em que o documento foi consultado. Qualquer outra informação, se conhecida (nomes de autor, datas, referência a uma publicação de origem, etc.), também deve ser dada.
Tamma PD, Heil EL, Justo JA, Mathers AJ, Satlin MJ, Bonomo RA. Infectious Diseases Society of America Antimicrobial-Resistant Treatment Guidance: Gram-Negative Bacterial Infections. Infectious Diseases Society of America 2024; Version 4.0. [accessed 22 July 2024] Available at: https://www.idsociety.org/practice-guideline/amr-guidance/.
Preprint:
Sonntag F, Sonntag K, Reißfelder C, Stange DE, Weitz J, Bogner A, et al. Early detection of anastomotic leakage in upper gastrointestinal surgery. Preprint at: medRxiv 2024.05.24.24307864; doi: https://doi.org/10.1101/2024.05.24.24307864.
7.4.2. Style
The PJS follows the AMA Manual of Style (11th Edition) and the ICMJE Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals (http://icmje.org/recommendations).
Footnotes
Footnotes should be avoided. When necessary, they must be numbered consecutively and appear at the bottom of the appropriate page.
Acknowledgments (optional)
Collate acknowledgements in a separate section at the end of the article before the references; to thank all of those who contributed to the study but have no weight of authorship. In this section, you can thank all of the sources of support, whether financial, technological or consulting, as well as individual contributions.
Abbreviations
Abbreviations should be used sparingly – only where they ease the reader’s task by reducing the repetition of long, technical terms. Initially use the word in full, followed by the abbreviation in parentheses. Thereafter use the abbreviation only. This is not needed if the abbreviation is a unit of measure.
Numbers
Numbers one through nine, must be written in length, except when they have decimals or if followed by units of measure. Numbers greater than nine are written in digits, except at the beginning of a sentence. The point is to be used as a decimal separator. A thousand separators should not be used.
Numeric ranges must be separated by "–" (for example, 25-30). A space between a value and the respective unit of measure should be used (for example, 25-30 mg), except for percentages (for example, 3%) and temperature values (for example, 5ºC), which must be presented without a space.
Units of Measure
Units of measure in the International System of Units should be used. Measures of length, height, weight and volume should be expressed in metric units (meter, kilogram, or liter) or their decimal multiples. Temperatures should be written in degrees Celsius (°C), blood pressure in millimeters of mercury (mmHg), and hemoglobin in g/dL. All measurements should be referred to in the biochemical or hematological metric system according to the International System of Units (SI).
Names of Diseases
The names of diseases should be written with lowercase initial letters, except for those that contain toponyms or anthroponyms.
Trade names
Chemical substances should be referred to by their generic name only. Trade names should not be used. Drugs should be referred to by their generic names. If proprietary drugs have been used in the study, refer to these by their generic name, mentioning the proprietary name, and the name and location of the manufacturer, in parentheses.
Names of instruments and equipment
Instruments of measurement, diagnosis or computer programs used in the study and mentioned in the manuscript should be presented in a general manner and by their commercial description, followed by the symbol ® and the name of the manufacturer, in parentheses.
Genes, mutations, genotypes and alleles
They must be written in italics. The recommended name should be consulted in a genetic nomenclature database (e.g. HUGO for human genes). Sometimes, it is advisable to indicate the gene synonyms the first time that they appear in the text. Gene prefixes such as those used for oncogenes or cellular localization should be shown in italics.
Tables and Figures
Tables should be self-contained and complement, but not duplicate, information contained in the text. Number tables consecutively in the text in Arabic numerals. Legends should be concise but comprehensive – the table, legend and footnotes must be understandable without reference to the text. Vertical lines should not be used to separate columns. Column headings should be brief, with units of measurement in parentheses; all abbreviations must be defined in footnotes. Footnote letters (a, b, c, d, etc.) must be used, not symbols and *, **, *** should be reserved for p-values.
Figures - All illustrations (line drawings and photographs) are classified as figures. Figures should be numbered using Arabic numerals, and cited in consecutive order in the text. Each figure should have a legend (figure title and other explanatory text).
For photographs of identifiable persons, the authors must obtain a signed consent for publication.
Each Figure and Table included in the manuscript must be referred to in the text: “An abnormal immune response can be at the source of the symptoms of the disease (Fig. 2). It is associated with the other two lesions (Table 1).”
Figure: When referred to in the text is abbreviated as Fig., while Table is not abbreviated. In captions, both words are written unabbreviated.
The submission must be made separately from the text following the instructions in the platform.
The figure files must be provided in high resolution, 1200 dpi for graphics or line art and 500 dpi for photographs and other images.
The color illustrations are published at no extra cost. Image files should be delivered in one of the following formats:
- JPEG (.jpg)
- Portable Document Format (.pdf)
- PowerPoint (.ppt)
- TIFF (.tif)
- Excel (.pptx)
All previously published and copyrighted material, including illustrations (figures and tables), must be accompanied by the written permission of the copyright owner and will have to present it in the submission.
Multimedia Files
The multimedia files must be sent in a separate file with the manuscript. The multimedia material must follow the quality standards of production for publication with no need for any modification or editing. Acceptable files include: MPEG, AVI or QuickTime formats.
Appendices
Appendices will be published along with the accepted article.
Appendices should be used to submit long or detailed surveys, extensive mathematical calculations and/or item lists. They should be placed after the reference list, if necessary, with captions.
If more than one appendix is present, they should be identified as A, B, etc. Formulas and equations in appendices must be numbered separately: (A.1), (A.2), etc.; the next appendix should be named, (B.1) and so on. Similarly, for tables and figures, they should be named: Table A. 1; Fig. 1; A.1, etc.
8. After Acceptance
8.1. Author Appeal Policy
Sometimes editors make mistakes. If an author believes that an editor has made an error in declining a paper, we welcome an appeal. In your appeal letter, which should be sent to editorial office editorchefe@spcir.com, please state why you think the decision is mistaken and set out your specific responses to any peer reviewers’ comments if those seem to have been the main cause of rejection.
The appeal will be considered by the Editor-in-chief and other relevant editors. Editorial decisions are rarely overturned. The PJS response to the appeal will be final. Even if the journal agrees to reconsider the manuscript, acceptance is not guaranteed, and the reconsideration process may involve previous or new reviewers or editors and substantive revision.
8.2. Correction Policy
8.2.1. Corrections
PJS publishes changes, amendments or retractions to a previously published article if, after publication, errors or omissions are identified which affect the interpretation of the data or information. Changes to published articles that affect the interpretation and conclusion of the article, but do not fully invalidate the article, will, at the Editor-in-chief discretion, be corrected via publication of a Correction linked to the original article.
8.2.2. Retractions
On rare occasions, when the interpretation or conclusion of an article is substantially undermined, it may be necessary for published articles to be retracted. PJS will follow the COPE guidelines in such cases. Retraction notices are linked to the original article. The original article is watermarked as retracted and the title is amended with the prefix “Retracted article:”
8.2.3. Editorial Expressions of Concern
When an Editor becomes aware of serious concerns regarding the interpretation or conclusion of a published article, they may choose to publish a statement alerting the readership. Scenarios in which Editorial Expressions of Concern may be published include prolonged investigations of very complex cases and when the concerns may have a significant and immediate impact on public health or public policy.
An Editorial Expression of Concern may be superseded by a subsequent Correction or Retraction but will remain part of the permanent published record.
8.2.4. Removal of Published Content
In exceptional circumstances, PJS reserves the right to remove an article, from the online platform. Such action may be taken when a content is defamatory, infringes a third party’s intellectual property right, right to privacy, or other legal right, or is otherwise unlawful; a court or government order has been issued, or is likely to be issued, requiring removal of such content; content, if acted upon, would pose an immediate and serious risk to health.
Removal may be temporary or permanent. Bibliographic metadata (e.g. title and authors) will be retained and will be accompanied by a statement explaining why the content has been removed.
8.3. Misconduct Handling Policy
PJS takes seriously allegations of potential misconduct. In cases of suspected research or publication misconduct, it may be necessary for the Editor-in-chief to contact and share manuscripts with third parties, for example, the author(s)’ institution(s) and ethics committee(s). PJS may also seek advice from COPE and discuss anonymized cases in the COPE Forum.
8.3.1. Research Misconduct
If there is suspicion that research has not taken place within an appropriate ethical framework, the Editor-in-chief may reject a manuscript and may inform third parties, for example, the author(s)’ institution(s) and ethics committee(s). In cases of proven research misconduct involving published articles, or where the scientific integrity of the article is significantly undermined, articles may be retracted.
8.3.2. Data Falsification and Fabrication
Data falsification is manipulating research data intending to give a false impression. This includes manipulating images, removing outliers or “inconvenient” results, changing, adding or omitting data points, etc. Data fabrication means the making up of research findings.
Any questions regarding data integrity raised during or after the peer review process will be referred to the Editor-in-chief. If the original data cannot be produced, the manuscript may be rejected or, in the case of a published article, retracted. Cases of suspected misconduct will be reported to the author(s)’ institution(s).
8.3.3. Publication Misconduct
PJHN will follow the COPE guidelines outlining how to deal with cases of potential publication misconduct.
8.3.4. Plagiarism
If plagiarism is identified, the COPE guidelines on plagiarism will be followed.
8.4. Complaint Policy
Complaints about our processes or publication ethics will in the first instance be handled by the Editor-in-chief.
For complaints about processes, such as time taken for review, the Editor will review and respond to the complainant's concerns.
For complaints about publication ethics or scientific content, the Editor will follow guidelines published by the Committee on Publication Ethics. The Editor then decides on a course of action and provides feedback to the complainant.
9. Publication Process After Acceptance
9.1. Accepted Articles
After a manuscript has been accepted for publication, it will be copyedited, and an electronic proof will be made available for author correction and approval. Authors will be notified by email when their proofs are ready.
9.2. Proof Reading
The proofs, edited by PJS technical and language polishing services, will be sent to the Authors before the article is published for approval of the layout and identification of any typographical errors. Corresponding authors are provided with proofs via email to check for technical editing, copyediting, and/or typesetting errors. At this stage, only minor corrections are permitted. Corrections should be returned within 48 hours. Failure to comply with the proposed deadline exonerates the PJS from accepting the revision by the Authors in any of these stages, and the revision can be carried out exclusively by the PJS services. After online publication, further changes can only be made in the form of an Erratum, which will be hyperlinked to the article.
9.3. Ahead of Print
The article will be published online after receipt of the corrected proofs. This is the official first publication citable with the DOI. After the release of the issue version, the paper can also be cited by issue and page numbers.
10. Post Publication
10.1. Promotion
Articles published in PJS are included in article alerts and regular email updates. Some may be highlighted on the Portuguese Society of Surgery homepage.
10.2. Access and Sharing
When the article is published online, the link to the published article will be shared through social media.
10.3. Author Interview
Your paper may be selected for a podcast. If so, the Web Editor will contact you to arrange a pre-recorded interview to discuss your paper.
11. Supplement Publication Policy
PJS will consider the publication of sponsored supplements that are of interest to its readers and demonstrate scientific validity. The content must be of sufficient informational value and quality to warrant a separate journal issue and must have a unifying theme. Submission of a supplement from a symposium or conference must occur in a timely fashion; in general, supplements will not be published if the publication date is more than 12 months after the event. No more than 2 supplements per month will be published. Publication costs must be borne entirely by the sponsor(s).
A written proposal for the supplement must be submitted to the Editor-in-Chief for consideration. The proposal must contain:
- The Guest Editor's or Coordinator's name, affiliation, and contact information;
- Topic(s) to be covered by the supplement, with a preliminary table of contents;
- If the supplement is to be based on a conference or symposium, information on dates, venue, and financial supporter(s);
- An estimate of the total number of double-spaced manuscript pages; and
- Sponsor(s) of the supplement.
A Guest Editor is a subject expert who is responsible for the content of the supplement, ensuring the quality of each component manuscript and its contribution to a cohesive, coherent whole. The Guest Editor is responsible for ensuring that all manuscripts are in final form before submitting. The Guest Editor may elect to write an introductory piece, but each article must be able to stand on its own. In the absence of a Guest Editor, the authors are fully responsible for ensuring that the articles are consistent with one another and that their manuscripts are in final form before submission. In such cases, a Coordinator is responsible for handling all submissions and facilitating communications between the authors and the editorial office.
PJS can provide information on the journal’s production schedule and can recommend deadlines for receipt of materials that are intended to allow enough time for review, revision, and reconsideration of the supplement manuscripts. However, any estimated publication date is simply a projection based on the information available at the outset; whether it can be met will depend on the submission of the completed manuscripts in a timely fashion, the nature of the review required, and the extent of mandatory revisions. Ideally, a supplement based on a conference or symposium should be planned so that authors provide the manuscripts to the Guest Editor or Coordinator at the time of the meeting.
The manuscripts must be prepared and submitted according to standards governing regular journal content.
All supplements will undergo an appropriate review of their contents.
Manuscripts will almost invariably require revision; in addition, the Editor-in-Chief reserves the right to reject portions of the supplement or the entire supplement. The editorial office will contact the Guest Editor or Coordinator regarding the decision to accept, reject, or require additional revisions. Once a supplement has been accepted it is formally scheduled for publication; changes to the publication date at this stage cannot be accommodated.
The supplement must contain a statement indicating the source(s) of funding.
Furthermore, the Guest Editor or Coordinator must state what, if any, financial relationship they may have with the sponsor of the supplement. Likewise, all authors should disclose what, if any, financial relationship they have with the sponsor of the supplement, or the manufacturer of any products (or competing products) that are discussed in their manuscripts. Each manuscript must list any support received. If medical writer(s)/editor(s) have been involved, their role must be explicitly acknowledged, and their affiliation/source of funding must be listed. Additionally, if the sponsor has a financial interest in a product either directly or indirectly discussed in the manuscript, this relationship should be identified, along with the name of the product. Information about sponsorship and related products will be published with each article in the supplement.
Articles published in a PJS supplement are subject to the same copyright restrictions that apply to articles published in regular journal issues.
Last updated September 2024
Original Papers
Are unpublished articles referring to research, casuistic, regarding clinical cases that have research on causes, mechanisms, diagnosis, evolution, prognosis, treatment or prevention of disease. The text can not exceed 5000 words and 50 bibliographic references. They will be submitted to peer review.Review Article
Editors encourage the submission of review articles or meta-analyzes on topics of interest. The text should not exceed 5,000 words in 5 pictures and 50 bibliographical references.
The Editors may request Review Articles, these being not subject to peer review and acceptance thereof for publication of the Editor in Chief's exclusive competence.
Letters to the Editor
In this section will be published letters to the editor.Opinion Article
In this section will be published articles with perspectives and opinions of the authors on medical education, ethics and medical deontology and other issues considered relevant. They should be structured with Summary and Keywords in Portuguese and English and should not exceed 2500 words, 2 images and 5 bibliographical references. They will not be submitted to peer review and their acceptance for publication is the sole responsibility of the Editor in Chief.
Clinical Case
Reports of Cases, preferably rare, didactic or that constitute unusual forms of presentation. They should not exceed 1800 words, 4 images and 15 bibliographical references. They will be submitted to peer review.
At the invitation of the editors, comments on the case may be published.
Technical Steps
Articles focusing on surgical technique or surgical procedures in which the authors present and describe particular aspects of the same with interest in their innovation, results and reproducibility. Limited to 5000 words and 25 bibliographical references. Without limit of illustrations, being the criterion of final choice determined by the Editor in Chief.Images for Surgeons
This section is intended for the publication of images (clinical, radiological, histological, surgical) related to surgical cases. The maximum number of pictures and pictures will be 3. The images should be of very good technical quality and didactic value. The text that may accompany the images should be limited to 250 words. They will be submitted to peer review.Recommended Guidelines
In this section will be published recommendations for clinical practice, preferably by groups or entities of reference in the clinical areas concerned.History and Careers
In this section will be published original articles relating to facts and historical figures for surgery in Portugal and the World. The text should not exceed 5000 words and 25 references. Will be submitted to peer review.
Special Notebook
Controversies
These are works prepared at the invitation of the Editors. They will relate to topics where there is no consensus and where there are opposing or markedly different positions. They will always be asked for 2 points of view, regarding opposing opinions. The text of each of the authors should not exceed 2500 words, 2 images and 10 bibliographical references.Recommended Readings
Section where summary reviews of books, multimedia material or other, that have particular relevance for scientific and technical updating will be published. Limited to 250 words and 1 illustration. Not subject to peer review.Fundamental texts
Texts that by their scientific importance, are considered by the Chief Editor as relevant to the knowledge in the field of surgery.Copyright Notice
Para permitir ao editor a disseminação do trabalho do(s) autor(es) na sua máxima extensão, o(s) autor(es) deverá(ão) assinar uma Declaração de Cedência dos Direitos de Propriedade (Copyright). O acordo de transferência, (Transfer Agreement), transfere a propriedade do artigo do(s) autor(es) para a Sociedade Portuguesa de Cirurgia.
Se o artigo contiver extractos (incluindo ilustrações) de, ou for baseado no todo ou em parte em outros trabalhos com copyright (incluindo, para evitar dúvidas, material de fontes online ou de intranet), o(s) autor(es) tem(êm) de obter, dos proprietários dos respectivos copyrights, autorização escrita para reprodução desses extractos do(s) artigo(s) em todos os territórios e edições e em todos os meios de expressão e línguas. Todas os formulários de autorização devem ser fornecidos aos editores quando da entrega do artigo.


